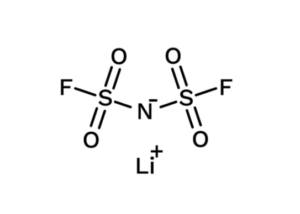
LiFSI( CAS 171611-11-3): High-Performance Electrolyte Salt
As EV and energy-storage markets push for higher energy density, faster charging, and wider operating windows, lithium bis(fluorosulfonyl)imide (LiFSI) has gained attention. Compared with LiPF₆, it offers higher ionic conductivity, stronger thermal and chemical stability, and more robust interphase formation.At Hainan Sincere Industries Co., Ltd., we provide high-purity LiFSI for R&D, pilot runs, and large-scale battery production.
What is LiFSI?
LiFSI (LiN(FSO₂)₂) is a lithium salt with a delocalized anion. It dissociates efficiently in carbonate, ether, and mixed solvent systems.
Its key benefits—highlighted in many academic and industrial studies—include:
-
High ionic conductivity over a broad temperature range.
-
Strong thermal stability with less hydrolysis compared to LiPF₆, reducing HF formation.
-
Stable SEI/CEI formation on anodes and high-voltage cathodes.
-
Compatibility with concentrated electrolyte systems (HCE/LHCE), improving transport and safety.
Key Application & Research Themes for LiFSI
High-voltage Li-ion batteries
LiFSI electrolytes can operate above 4.3–4.5 V when paired with stabilizing solvents like FEC, EMC, or fluorinated carbonates. Film-forming additives further strengthen the cathode–electrolyte interphase (CEI).As a result, transition-metal dissolution is reduced, and impedance growth is minimized during long cycling.
Fast-charging systems
Due to its strong dissociation and high Li⁺ mobility, LiFSI lowers polarization at high C-rates. In EV tests with 3C–6C pulses, blends maintain capacity and limit lithium plating when combined with SEI-forming additives.
Low-temperature operation
LiFSI enhances battery performance in sub-zero environments (−20 to −40 °C). This is especially true in ether-rich or fluorinated carbonate mixtures. Many researchers combine it with low-viscosity solvents to cut internal resistance in cold climates.
Silicon-rich anodes
High-capacity silicon anodes (Si/C, SiOx) benefit from LiFSI-based electrolytes. When paired with additives such as FEC, VC, or LiDFOB, LiFSI forms elastic, inorganic-rich SEIs. These layers accommodate volume changes and help avoid particle cracking.
Lithium-metal & “anode-free” cells
In lithium-metal research, LiFSI is key for high-concentration and localized high-concentration electrolytes. Formulations with ether/carbonate and non-solvating diluents like HFE promote dense lithium plating and reduce dendrites.
High-concentration electrolytes (HCE/LHCE)
LiFSI shifts the solvation structure toward anion coordination. This produces LiF-rich and sulfate-containing interphases that withstand high voltage, high temperature, and abuse. Both graphite and lithium-metal cells show benefits in this system.
Polymer, gel, and ionic-liquid hybrids
it dissolves easily in PEO, PVDF-HFP gels, and ionic liquids. These systems create non-volatile, flame-retardant, or quasi-solid electrolytes for safer cells and flexible electronics.
Safety & abuse tolerance
With optimized solvents, it reduces gas evolution, lowers flammability, and improves heat tolerance. This is valuable for EV and stationary storage systems facing strict safety standards.