What is benzenesulfonic acid?
Benzenesulfonic acid, a strong organic sulfonic acid, is widely utilized in pharmaceuticals, dyes, water treatment, and industrial catalysis. Available as a white to light-yellow crystalline powder with purity grades of 99%, 98%, 95%, and 93%, it offers versatility for diverse industrial requirements. Its high solubility in water, ethanol, and ether ensures efficient reactivity as a key intermediate.
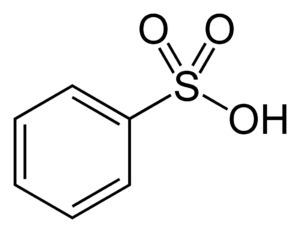
Benzenesulfonic Acid also known as Benzenemonosulfonic acid,phenylsulfonic acid, acide benzenesulfonique, benzene sulphonic acid and besylic acid. Sulfonic acid(RSO2OH), where R is an aliphatic or aromatic hydrocarbon.
It is a derivative of sulfuric acid (HOSO2OH) where an OH has been replaced by a carbon group or a compound where a hydrogen atom has been replaced by treatment with sulfuric acid; for example, benzene is converted to Benzenesulfonic Acid.
Benzenesulfonic acid structure
FAQs:
1. What is benzenesulphonic acid used for?
Benzenesulfonic acid is used to produce phenol by fusing it with sodium hydroxide or hydrolyzing one of its salts, usually sodium. Other uses include surfactants made with its metal or amine salts and as a counterion for cationic pharmaceuticals
- Pharmaceuticals: Intermediate for antibiotics, antiviral drugs, and sulfonamides.
- Dye Manufacturing: Acid dye synthesis and textile auxiliaries.
- Water Treatment: Functional group source for ion-exchange resins.
- Catalysis: Acid catalyst in esterification and alkylation reactions.
- Polymer Production: Used in sulfonation processes for specialty polymers.
- In cleaning: Household cleansers including laundry maquillages, laundry liquids, dishwashing liquids and other ménage cleansers. It’s used in anionic specialty phrasings in other diligence similar as cloth diligence. It is used as a mercerizing or washing agent.
2. Is benzenesulfonic acid harmful?
This produces toxic and corrosive fumes. The solution in water is a strong acid. It reacts violently with bases and is corrosive. Reacts violently with oxidants.
UN NO.:2585
Primary hazard class for transport: Class 8
Packaging type: III
UN proper shipping name: Alkyl sulfonic acids, solid, containing not more than 5% free acid
3. Benzoic acid and benzenesulfonic acid, Which is stronger?
To determine which one is stronger, try looking up Ka or pKa data for the two. The pKa for benzenesulfonic acid is -2.8, while benzoic acid has a pKa of 4.2. This means that benzenesulfonic acid will ionise to a greater extent, leaving protons (H+) and the conjugate base (C6H5SO3 -), with negligible amounts of the original, unionised acid remaining. Benzoic acid is a much weaker acid, which will only ionise to a small degree, and largely remain as the unionised acid molecule in solution.
Benzene sulphonic acid is more acidic than benzoic acid as after losing acidic proton, we’re left with conjugate base. More stable the conjugate base, more will be the acidic strength.
Considering benzene sulphonic acid, it has 3 equivalent resonance structures i.e charge can delocalise over 3 oxygens as compared to benzoic acid which has 2 oxygens and thus 2 equivalent resonance structures.
Benzoic acid is the caroboxy acid of benzene with a COOH group. Benzene sulfonic acid is the product of reaction of Sulfuric acid with benzene, Sulfuric aid is stronger than Carbonic acid. henec the benzene sulfonic acid is certainly a stronger acid.