Sugar Encyclopedia | Activity and application of chitosan and its derivatives
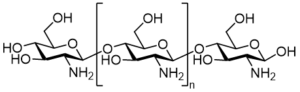
Chitin is deacetylated under alkaline conditions and is called chitosan when a certain degree of deacetylation is reached. Therefore, chitosan is also known as deacetylated chitosan. Chitosan is a natural linear polysaccharide composed of multiple N-acetyl-D-glucosamine monomers connected by β-1,4-glycosidic bonds. The structure is shown in Figure 1. Chitosan is widely present in the shells of crustaceans such as insects, shrimps and crabs, as well as in the cell walls of fungi. It is the second most abundant polysaccharide in nature after cellulose. Due to its significant antibacterial properties and the advantages of non-toxicity, biodegradability and biocompatibility, it has attracted widespread attention in recent years. Chitosan has antibacterial, anti-inflammatory, anti-tumor, antioxidant, procoagulant, wound healing, blood sugar lowering, immunomodulatory, neuron protection and other effects. It has potential application value in the treatment of diseases such as tumors, diabetes, obesity and hypertension [1].
Antimicrobial activity
Among the various properties of chitosan, the antimicrobial effect of chitosan and its application have always been the focus of many studies. Chitosan has limited solubility in aqueous media above pH 6 and only exhibits antimicrobial properties in acidic media. Due to the lack of positively charged amino groups and low solubility in aqueous media, this activity is not observed under high pH conditions. Therefore, the antimicrobial activity of chitosan derivatives with other groups has attracted much attention.
Sahariah et al. [2] introduced quaternary ammonium groups with permanent positive charges on the chitosan backbone. The analysis results showed that N, N, N-trimethyl chitosan with a quaternization degree of 100% had the highest antimicrobial activity, moderate cytotoxicity, generally good water solubility, and significant antimicrobial activity at neutral pH 10.
Although antimicrobial chitosan compounds have been widely studied, their mechanism of action and structure-activity relationship remain largely unclear due to the multiple influencing parameters involved. Geisberger et al. [3] introduced chitosan-thioglycolic acid (LMW-TGA) as a new type of antibacterial agent, and conducted comparative tests on its antibacterial activity with trimethyl chitosan (TMC) and carboxymethyl chitosan (CMC). They found that it has high antibacterial activity against Gram-positive bacteria, Gram-negative bacteria and fungi, and can produce antibacterial activity at neutral and physiologically relevant pH values.
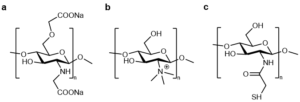
In order to improve the antibacterial and antioxidant properties of chitosan, Tamerd et al. [4] prepared N-cinnamoyl substituted O-amine functionalized chitosan, the structure of which is shown in Figure 3. The primary amine group of O-amine functionalized chitosan was coupled with the aldehyde group of cinnamaldehyde to obtain the corresponding Schiff base of N-cinnamoyl substituted O-amine functionalized chitosan. The antibacterial property evaluation showed that the antibacterial activity of the new Schiff base generated from O-amine functionalized chitosan was better than that of chitosan and O-amine functionalized chitosan.
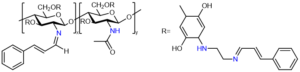
Pro-coagulation and wound healing
A higher degree of deacetylation can improve the aggregation of red blood cells and platelets, which is necessary for promoting coagulation. When N-acetyl-D-glucosamine is deacetylated to form D-glucosamine, it has free amino groups in its molecular structure, and chitosan acquires a positive charge. This cationic property is used to promote coagulation. This is because the surfaces of platelets and red blood cells are negatively charged due to the presence of phosphatidylcholine, phosphatidylethanolamine, and sialic acid groups, respectively, which cause electrostatic repulsion between them and hinder the aggregation process [5].
Coagulation is the first stage in the effective control of bleeding during wound healing. The purpose of coagulation can be achieved by using porous dressings, such as alginate and chitosan. Products made of pure chitosan may dissolve when used in vivo, while the combination of chitosan and carrageenan polymers will overcome this limitation and can be used for wound treatment. Biranje[6] et al. used ion complexation to prepare a complex of anionic carrageenan and cationic chitosan. The complex has enhanced hemostatic activity, enhanced cell adhesion and aggregation of red blood cells and platelets, and is non-toxic on the surface. It is a bleeding wound healing material.
Rao[7] et al. used mushroom carboxymethyl chitosan (NAM-CMCS) as a natural polymer stabilizer to prepare ZnO nanocomposites by ultrasound at room temperature and characterized them. Compared with ordinary carboxymethyl chitosan, NAM-CMCS-ZnO nanoparticles have better biocompatibility and anticoagulant properties, and have synergistic antibacterial activity, and have good prospects in wound dressings.
Hydrogels are similar to biological tissue structures, have good biocompatibility and adaptability, and have been widely used in the medical field. Wang[8] et al. prepared a medical injectable hydrogel composed of polylysine (ε-PL) and carboxymethyl chitosan (CMCS), which has the characteristics of rapid gelation, low toxicity, low irritation, and injectability. The principle is shown in Figure 4.

Anti-tumor
According to a report released by the World Health Organization (WHO), cancer is the second leading cause of death in the world. Different treatment strategies have been proposed for cancer treatment, such as surgery, radiotherapy, chemotherapy, immunotherapy, etc. Although these methods have achieved significant results in cancer treatment, they have a variety of side effects on patients due to the lack of specific and targeted drug delivery. Chitosan molecules contain free hydroxyl and amino groups and are easy to be chemically modified. They can not only be coupled with anti-tumor drugs by ester bonds or amide bonds, but also be connected with ligands or antibodies that specifically bind to the corresponding receptors on the surface of tumor cells to prepare active targeted anti-tumor drug carrier systems. In addition, chitosan’s good film-forming properties and bioadhesion can be used to prepare anti-tumor drug microparticles to increase drug absorption and reduce the toxic side effects of drugs[9]. Therefore, chitosan is an anti-tumor carrier with good development prospects.
Sabzevar[10] et al. prepared chitosan nanoparticles (CM11) by ion gel method, and used EDC as coupling agent to bond hyaluronic acid carboxyl groups to chitosan amine groups, thus obtaining hyaluronic acid-coated chitosan nanoparticles (HA-CS NPs). Experiments showed that CM11-loaded HA-CS nanoparticles had a significant killing effect on tumor cells. Dragostin[11] et al. designed a new anti-angiogenic chitosan nanomaterial. The chitosan derivative nanostructure had stronger antitumor activity than chitosan alone. Guo[12] et al. prepared hydroxycamptothecin nanoparticles (NPs/HCPT), characterized them, and tested their antitumor activity in vivo. The results showed that NPs/HCPT could significantly inhibit the growth of melanoma cells.
Antioxidant
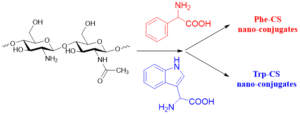
Chitosan and its derivatives also have excellent antioxidant activity. By activating antioxidant enzymes in the body, they can enhance the body’s ability to scavenging oxygen free radicals. Beyazit et al. [13] synthesized two new chitosan-gossypol derivatives (HCS-GSP and LCS-GSP) by condensing high molecular weight chitosan and low molecular weight chitosan with (-)-gossypol, respectively. The scavenging ability of these two chitosan-gossypol derivatives for DPPH free radicals is better than that of unmodified chitosan. Wang [14] designed and developed aromatic amino acid-coupled chitosan using a multispectral method, and successfully grafted tryptophan (Trp) and phenylalanine (Phe) onto chitosan (CS) to form Trp-CS and Phe-CS nanocomposites, as shown in Figure 5. Antioxidant experiments showed that both Trp-CS and Phe-CS have good antioxidant activity.
Hypoglycemic
Chitosan has the function of regulating blood glucose concentration, and can reduce and delay blood glucose peaks. It can also adjust the human body’s pH to a weak alkaline state, thereby increasing insulin utilization. Chitosan nanoparticles have received widespread attention in oral insulin delivery by targeting their binding to molecules such as folic acid and peptides [15]. The insulin-loaded folic acid-chitosan (FA-CS) nanoparticles prepared by Leithy [16] et al. significantly improved oral hypoglycemic activity, increasing their potential as oral insulin carriers. Prabahar [17] et al. prepared thiolated chitosan nanoparticles, which can enhance the adhesion of sitagliptin to the gastrointestinal tract, prolong drug release time, reduce side effects, and improve patient compliance.
Other activities
Although chitosan has a wide range of medical application potential, its differences in immunoreactivity limit its clinical application. Ravindranathan [18] et al. explored the direct effects of chitosan on immune responses at the cellular level and were able to separate chitosan-specific immune responses from more complex processes such as foreign body reactions and inflammatory responses that occur after in vivo injection. Chitosan and its derivatives, as a bioactive compound extracted from the ocean, have been reported to have good neuroprotective effects, such as β-amyloid protein and acetylcholinesterase inhibitory activity, anti-neuroinflammatory and anti-apoptotic effects [19]. In addition, chitosan is widely used in drug carriers. Mozafari [20] prepared and characterized a chitosan system for the combined delivery of simvastatin and citicoline to overcome the adverse side effects of simvastatin in Alzheimer’s disease.
Summary and Outlook
The popularity of chitosan is due to its versatility, availability and biocompatibility. Chitosan has antibacterial, procoagulant and wound healing effects, anti-tumor, antioxidant, hypoglycemic, immunomodulatory, and neuroprotective effects. According to structure-activity studies, structural modification of chitosan and introduction of other groups can enhance its corresponding activity. Chitosan is often used to develop new drug delivery systems and controlled release platforms. It is soluble in moderately acidic aqueous solutions and is therefore easy to formulate with various biopharmaceuticals. Chitosan and chitosan derivatives in the form of hydrogels are also under study and are a promising material.
References
[1] Liu Wen, Tong Qiaoyun. Research progress and medical application value of chitosan and its derivatives. Bachu Medicine, 2018, Vol. 1, No. 4: 115–119.
[2] Sahariah, P. et al. The effect of substituent, degree of acetylation and positioning of the cationic charge on the antibacterial activity of quaternary chitosan derivatives. Mar. Drugs 2014, 12, 4635–4658.
[3] Geisberger, G. et al. Chitosan-thioglycolic acid as a versatile antimicrobial agent. Biomacromolecules. 2013, 14, 1010–1017.
[4] Tamer, T. M. et al. Antibacterial and antioxidative activity of O-amine functionalized chitosan. Carbohydr. Polym. 2017, 169, 441–450.
[5] Khan,M. A. & Mujahid, M. A review on recent advances in chitosan based composite for hemostatic dressings. Int. J. Biol. Macromol. 2019, 124, 138–147.
[6] Biranje,S. S. et al. Cytotoxicity and hemostatic activity of chitosan/carrageenan composite wound healing dressing for traumatic hemorrhage. Carbohydr. Polym. 2020,239, 116106.
[7] Rao,K. M., Suneetha, M., Park, G. T., Babu, A. G. & Han, S. S. Hemostatic,biocompatible, and antibacterial non-animal fungal mushroom-based carboxymethylchitosan-ZnO nanocomposite for wound-healing applications. Int. J. Biol.Macromol. 2020, 155, 71–80.
[8] Wang,Y., Cao, H. & Wang, X. Synthesis and characterization of an injectableε-polylysine/carboxymethyl chitosan hydrogel used in medical application. Mater.Chem. Phys. 2020, 248, 122902.[9] Yuan-zheng,Z. A. W. X. F. The Research Advance on the antitumor effect of chitosan and itsderivatives *. 2020, 10, 992–994.
[10] Taghipour-Sabzevar,V. et al. Targeted delivery of a short antimicrobial peptide against CD44-overexpressing tumor cells using hyaluronic acid-coated chitosan nanoparticles: An in vitro study. J. Nanoparticle Res. 2020, 22.
[11] Dragostin,O. M. et al. Designing of Chitosan Derivatives Nanoparticles with Antiangiogenic Effect for Cancer Therapy. Nanomaterials. 2020, 10.
[12] Guo,H. et al. Preparation and Characterization of Chitosan Nanoparticles for Chemotherapy of Melanoma Through Enhancing Tumor Penetration. Front. Pharmacol. 2020, 11, 1–8.
[13] Beyazit,N., Çakran, H. S., Cabir, A., Akışcan, Y. & Demetgül, C. Synthesis,Characterization and antioxidant activity of chitosan Schiff base derivatives bearing (-)-gossypol. Carbohydr. Polym. 2020, 240.
[14] Wang,Y. et al. Self-assembly, rheological properties and antioxidant activities of chitosan grafted with tryptophan and phenylalanine. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 597, 124763.
[15] Agrawal,A. K., Urimi, D., Harde, H., Kushwah, V. & Jain, S. RSC Advances the stability, bioavailability and efficiency of insulin in. 2015, 5, 105179–105193.
[16] ElLeithy, E. S., Abdel-Bar, H. M. & Ali, R. A. M. Folate-chitosan nanoparticles triggered insulin cellular uptake and improved in vivo
hypoglycemic activity. Int. J. Pharm. 2019, 571, 118708.
[17] Prabahar,K., Udhumansha, U. & Qushawy, M. Optimization of thiolated chitosan nanoparticles for the enhancement of in vivo hypoglycemic efficacy of sitagliptin in streptozotocin-induced diabetic rats. Pharmaceutics. 2020, 12.[18] Ravindranathan,
S., Koppolu, B. P., Smith, S. G. & Zaharoff, D. A. Effect of chitosan properties on immunoreactivity. Mar. Drugs. 2016, 14.
[19] Hao, C., Wang, W., Wang, S., Zhang, L. & Guo, Y. An overview of the protective effects of chitosan and acetylated chitosan oligosaccharides against neuronal disorders. Mar. Drugs. 2017, 15.[20] Mozafari, N., Farjadian, F., Mohammadi Samani, S., Azadi, S. & Azadi, A. Simvastatin-chitosan-citicoline conjugates nanoparticles as the co-delivery system in Alzheimer susceptible patients. Int. J. Biol. Macromol. 2019, 14, 233-240.







